
En tant que Fabricant de produits chimiques HPMCJ'ai toujours été fasciné par la structure des composés et la façon dont elle affecte leurs propriétés. L'un de ces composés a attiré mon attention : l'hydroxypropylméthylcellulose (HPMC). La formule de l'HPMC est C56H108O30. Dans ce guide complet, je vous présenterai la structure chimique de l'HPMC, ses applications, ses propriétés et la manière dont elle affecte ses performances.
1. Quelle est la structure chimique du HPMC ?
Le HPMC est un polymère semi-synthétique dérivé de la cellulose. Il est fabriqué en traitant la cellulose avec une solution alcaline pour former de la cellulose alcaline. La cellulose alcaline réagit ensuite avec du chlorure de méthyle et de l'oxyde de propylène pour former l'HPMC. La structure chimique de l'HPMC est complexe, mais on peut la simplifier en disant qu'il s'agit d'un squelette de cellulose avec des substituants méthyle et hydroxypropyle.
Le degré de substitution (DS) de l'HPMC correspond au nombre moyen de groupes hydroxypropyle et méthyle par unité d'anhydroglucose dans le squelette de la cellulose. Le DS peut varier de 0,1 à 2,5 et influe sur les propriétés de l'HPMC. Un DS plus élevé donne un polymère plus hydrophile avec une température de gélification plus basse et une meilleure résistance à la corrosion. solubilité dans l'eau.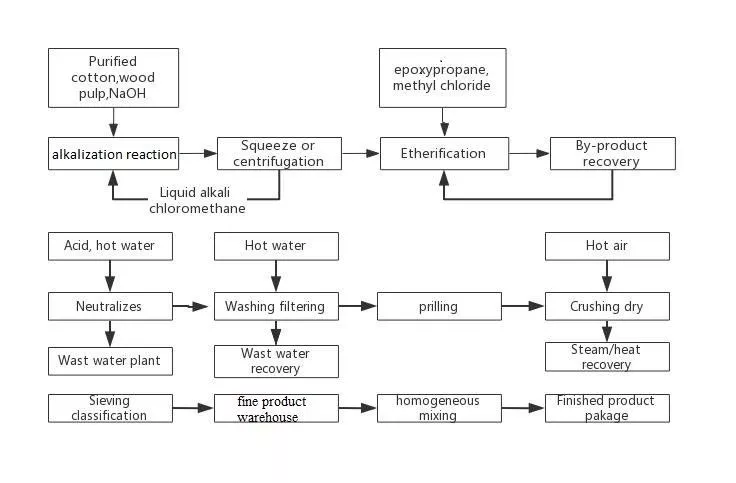
2. Applications du HPMC en fonction de sa structure chimique
Le HPMC a une large gamme d'applications dans diverses industries, y compris les produits pharmaceutiques, les produits alimentaires et les cosmétiques, détergent et la construction. Ses applications reposent sur ses propriétés uniques, qui résultent de sa structure chimique.
Dans l'industrie pharmaceutique, le HPMC est utilisé comme liant, désintégrant et agent de libération contrôlée dans les formulations de comprimés. Sa viscosité élevée et sa bonne rétention d'eau en font un produit idéal pour les solutions ophtalmiques et les sprays nasaux. La structure chimique du HPMC en fait également un excellent agent mucoadhésif, qui améliore l'absorption et la biodisponibilité des médicaments.
Dans l'industrie alimentaire, le HPMC est utilisé comme épaississant, émulsifiant et stabilisant. Sa structure chimique lui permet de former des gels et d'améliorer la texture des produits alimentaires. Le HPMC est également utilisé dans les produits alimentaires à faible teneur en matières grasses et sans sucre, car il peut imiter la texture et la sensation en bouche des matières grasses et du sucre.
HPMC de qualité construction est utilisé comme agent de rétention d'eau dans les formulations de ciment et de mortier. Colle à carreaux est le champ le plus important. Sa structure chimique lui permet de former un film protecteur autour des particules de ciment, ce qui empêche la perte d'eau et améliore la maniabilité.
Dans les détergents, le HPMC est utilisé comme épaississant et comme agent anti-précipitant. Sa structure chimique lui permet de fournir une meilleure viscosité et d'empêcher la saleté de se redéposer.
3. Comment la structure chimique affecte les performances du HPMC
La structure chimique de l'HPMC influe sur ses performances de diverses manières. Le degré de substitution affecte la solubilité, la température de gélification et la viscosité de l'HPMC. Un DS plus élevé donne un polymère plus hydrophile, ce qui augmente sa solubilité dans l'eau. Il réduit également la température de gélification de l'HPMC, ce qui facilite la formation de gels.
Le poids moléculaire du HPMC influe également sur ses performances. Un poids moléculaire plus élevé donne un polymère plus visqueux avec de meilleures propriétés de rétention d'eau. Il augmente également les propriétés mucoadhésives de l'HPMC, ce qui améliore l'absorption et la biodisponibilité des médicaments.
Le rapport entre les groupes méthyle et hydroxypropyle dans l'HPMC affecte ses propriétés de gélification. Un rapport plus élevé entre les groupes méthyle et hydroxypropyle se traduit par une vitesse de gélification plus rapide et un gel plus dur.
4. Propriétés du HPMC en fonction de sa structure chimique
La structure chimique du HPMC lui confère des propriétés uniques qui le rendent adapté à diverses applications. Voici quelques-unes des propriétés de l'HPMC
1. Solubilité : le HPMC est soluble dans l'eau et forme des solutions claires.
2. Viscosité : L'HPMC a une viscosité élevée, qui augmente avec le poids moléculaire et le DS.
3. Mucoadhésion : Le HPMC est un bon agent mucoadhésif, qui améliore l'absorption et la biodisponibilité des médicaments.
4. Gélification : L'HPMC peut former des gels à de faibles concentrations et à des températures élevées.
5. Rétention d'eau : Le HPMC possède de bonnes propriétés de rétention d'eau, ce qui permet de l'utiliser dans les solutions ophtalmiques et les sprays nasaux.
5. Comparaison de la structure chimique du HPMC avec d'autres éthers de cellulose
Le HPMC est l'un des nombreux éthers de cellulose utilisés dans diverses industries. Chaque éther de cellulose a une structure chimique unique qui influe sur ses propriétés et ses applications. L'HPMC se distingue des autres éthers de cellulose par son degré de substitution et le rapport entre les groupes méthyle et hydroxypropyle.
La méthylcellulose (MC) a un DS inférieur à celui de l'HPMC, ce qui la rend moins hydrophile et moins soluble dans l'eau. La MC forme également des gels plus faibles que l'HPMC en raison de la proportion plus faible de groupes hydroxypropyle.
Éthylcellulose (EC) a un DS plus élevé que le HPMC, ce qui le rend plus hydrophobe et moins soluble dans l'eau. L'EC est utilisé comme revêtement dans l'industrie pharmaceutique en raison de ses bonnes propriétés de formation de film.
6. Facteurs affectant la structure chimique du HPMC
Plusieurs facteurs peuvent affecter la structure chimique de l'HPMC, notamment la concentration des réactifs pendant la synthèse, le temps de réaction et la température de réaction. Le DS de l'HPMC peut également être modifié en utilisant différents ratios de chlorure de méthyle et d'oxyde de propylène.
7. Techniques analytiques pour l'étude de la structure chimique du HPMC
Plusieurs techniques analytiques peuvent être utilisées pour étudier la structure chimique de l'HPMC. Il s'agit notamment de la spectroscopie de résonance magnétique nucléaire (RMN), de la spectroscopie infrarouge à transformée de Fourier (FTIR) et de la chromatographie d'exclusion de taille (SEC).
La spectroscopie RMN peut être utilisée pour déterminer la DS de l'HPMC et le rapport entre les groupes méthyle et hydroxypropyle. La spectroscopie FTIR permet d'identifier les groupes fonctionnels de l'HPMC et de suivre les modifications de sa structure chimique. La SEC peut être utilisée pour déterminer le poids moléculaire et la polydispersité de l'HPMC.
8. Poids moléculaire et viscosité du HPMC
Le poids moléculaire et la viscosité de l'HPMC sont des paramètres importants qui affectent ses performances. Le poids moléculaire de l'HPMC peut être déterminé par SEC, tandis que la viscosité peut être mesurée à l'aide d'un viscosimètre.
Le HPMC de poids moléculaire élevé a une viscosité plus élevée et de meilleures propriétés de rétention d'eau. Cependant, il peut également entraîner une libération plus lente du médicament et une vitesse de dissolution plus lente. Le poids moléculaire de l'HPMC peut être contrôlé en ajustant les conditions de réaction lors de la synthèse.
Conclusion
En conclusion, la structure chimique de l'HPMC est complexe, mais elle lui confère des propriétés uniques qui le rendent adapté à diverses applications. Le degré de substitution, le poids moléculaire et le rapport entre les groupes méthyle et hydroxypropyle affectent les propriétés de l'HPMC, notamment sa solubilité, sa viscosité et ses propriétés de gélification. Des techniques analytiques telles que la spectroscopie RMN, la spectroscopie FTIR et la SEC peuvent être utilisées pour étudier la structure chimique de l'HPMC. Le poids moléculaire et la viscosité de l'HPMC sont des paramètres importants qui affectent ses performances. En tant que chimiste, je trouve la structure chimique de l'HPMC fascinante et j'espère que ce guide complet vous a aidé à mieux la comprendre.
CTA : Pour en savoir plus sur le HPMC et ses applications nous contacter aujourd'hui.


Laissez une réponse